GMP certification, GMP location and sale of the last plants
Dear Cannerald and CannerGrow Community,
In today's blog article we give you the long-awaited update on the progress and current status of the GMP (Good Manufacturing Practice) certification.
In addition, for the first time you will receive the complete roadmap of the certification and the associated path from CBD to THC cultivation
The blog article is divided into the following points:
1) Summary of the GMP certification
2) All details about the GMP certification
3) GMP location
4) Sale of the last plants
5) Laboratory area
🌱 1) Summary of the GMP certification
Since the beginning, one of Cannerald's most important goals has been to obtain GMP certification as quickly as possible in order to produce the highest quality medicinal THC cannabis for the pharmaceutical market.
As planned and announced by us from the beginning, CBD cannabis is only to bridge the gap until we are allowed to grow and sell medicinal THC cannabis.
In addition to our state-of-the-art production, which is already known for our exceptionally high quality, the future GMP certification is one of the other main features that differentiates us from other cannabis companies.
The three key features of unbeatable Cannerald quality in the very near future:
1. Our uniquely pure and high quality production (GACP) in which we strictly adhere to SOPs (Standard Operating Procedures) in order to exceed the GACP requirements
2. our own research facility with two laboratories in Fraubrunnen, biology and biochemistry laboratory (GMP)
3. our clean room area (GMP), more on that later
Our unbeatable advantage: Everything from one source from Cannerald, everything is produced, dried, trimmed, released and processed in-house and not outsourced to third parties.
This starts with the cutting, which is cut from our own mother plants, to the dried flowers, which are prepared for sale in the GMP clean room and the subsequent batch release by our own laboratories.
GMP - Step 1:
On July 7th, 2021, we submitted an application to the Swiss Federal Office of Public Health (FOPH) for a special permit for the cultivation of cannabis with more than one percent THC for scientific research.
The application was approved in March 2022 and at the end of May 2022 we received the first THC seeds approved by the BAG and SwissMedic for the cultivation of THC cannabis plants for scientific research.
Once the equipment from our two laboratories has been validated and approved by the manufacturers, we can start growing THC cannabis for scientific research.
This is an enormous advantage for us, because it allows us to optimally focus on the cultivation of medical THC cannabis and grow THC mother plants before the full three GMP steps are completed.
GMP - Step 2:
On August 2nd, 2022, on the first possible day for submitting an application for approval for the cultivation and trading of BetmG (narcotic drugs) to the BAG and Swissmedic, we submitted our application with all the necessary documents and papers.
According to the current status of the authorities, we expect an audit (acceptance) between January and February 2023.
GMP - Step 3:
The GMP clean room area is then approved by the RHI (Regional Medicines Inspectorate). If this is accepted, it is confirmed that we meet all the requirements for GMP certification, so in combination with step 2 we are allowed to produce and sell medicinal THC cannabis.
The completion of the first GMP clean room area in Fraubrunnen, which is sufficient for the Fraubrunnen site, will be between March and May 2023 (more details, see below).
Once these three steps have been taken, we are allowed to produce, process and sell medical THC cannabis internationally.
Only once we have received the GMP certification (step 3) will it be possible to convert the cannabis plants from CBD to medicinal GMP THC.
The switch from CBD to medical GMP THC cannabis is done grow room by grow room in order, starting with grow room Alpha.
These were the most important points in a nutshell on the subject of GMP.
We are currently on the home stretch for GMP and medicinal THC Cannabis by Cannerald.
🌱 2) All details about the GMP certification
For everyone from the Cannerald and CannerGrow community who wants to know more details, we have attached a more detailed version of the GMP history:
In order to be able to produce medical THC cannabis, we currently have two consulting companies specializing in GMP, one German and one Canadian. They support our team on site in every respect. For example, with the respective changes to the EU regulations, since it is a new and unknown market, as well as with SOPs for the respective work processes for GMP certification and also in our GACP area (production).
Important information:
The authorities do not give you a blueprint for the GMP clean room area.
It must always comply with the current regulations and the RHI (Regional Medicines Inspectorate) only decides after it has been built and during the audit whether to receive GMP certification or not.
If the regulations change, the GMP clean room area must also be adapted to the new regulations within a certain period of time, otherwise the GMP certification will be withdrawn.
Q3 2019 - GMP Precautions and Induction:
From Q3 2019 to Q2 2020, the first precautions were taken for GMP certification and familiarization with the topic of GMP. The team for the GMP certification was then set up.
Q2 2020 - GMP planning in Fraubrunnen:
From Q2 2020, the GMP clean room area in Fraubrunnen was planned. Most of the GMP cleanroom area was to be built where the Cannerald Event 2021 took place.
On the first day of construction, we imposed a construction freeze because new GMP regulations (EU-GMP INDEX 7) were published and we had to replan again so that the GMP clean room area corresponds to the new regulations.
The changes concerned the EU-GMP Index 7 “Manufacture of herbal medicinal products”. The focus here is on the definition: "From when/where does the GMP clean room area begin".
There was no clear regulation in 2020/2021 and we received different information from different sides.
Since we were already planning our second production site in Solothurn, Zuchwil and our expansion has been progressing for some time, we decided to relocate the GMP clean room area to our second production site in Solothurn, Zuchwil. This should be built taking into account the new regulations (EU-GMP INDEX 7) and be large enough for both locations.
Q4 2021 - GMP planning Solothurn, Zuchwil:
The planning of the GMP clean room area at our second production site in Solothurn, Zuchwil, started at top speed from Q4 2021, according to the new EU-GMP INDEX 7 regulations, as well as a significantly larger GMP clean room area.
The room books (URS - User requirement specification) for the GMP clean room area were written and preparations were made regarding the qualification of the clean room equipment.
2022: The planning was completed and construction was just around the corner (Q2 2022).
We received information again that a leading German GMP consulting company called ECA Academy (ECA) gave new recommendations for the production of medicinal cannabis (EU-GMP Index 7) to the German authorities. The recommendations defined when/where exactly the first GMP step must begin. Previously, it had not been clear whether it had to be during drying, at trimming or when the flowers were processed.
This is a huge cost differential in building the GMP clean room area as if all steps have to be GMP compliant then given the size of our two production sites and the volume of our resulting cannabis crops this would cost several millions more.
However, if we build the GMP clean room area and the regulations are changed, we lose the GMP certification within a certain period of time and have to carry out conversion work, which would possibly cost our clean room area several millions, if a conversion would be possible at all.
Q2/Q3 2022 - GMP planning Solothurn, Luterbach (external location):
Since the GMP clean room area is associated with considerable costs and we now finally know what the new regulations will be like, we have decided to move the clean room area to a external location.
The duration for the construction of the GMP clean room area including the audit will take about 1.5 years until the end of Q1 2024.
Due to the enormous importance of the GMP clean room area for Cannerald and CannerGrow, we bought our own hall for CHF 8.65 million through our group structure. The money for the purchase of the hall came from companies in our holding group and not from capital already planned for the construction, grow rooms and infrastructure from the pre-sale of plants.
GMP planning - final: Fraubrunnen & Solothurn, Luterbach:
Since we do not want to wait another 1.5 years until the final GMP clean room area is completed at the new location (construction + audit), we have developed a plan B in Fraubrunnen.
A clean room area is also being built in Fraubrunnen, with which we can meet the current EU-GMP regulations.
The completion of the first GMP clean room area in Fraubrunnen, which is sufficient for the Fraubrunnen site, will be between March and May 2023 (Completion of construction + GMP certification).
As soon as the new GMP clean room area, even larger than in Solothurn, Zuchwil, is planned to be built at Cannerald's new location in about 1.5 years, it will replace the first clean room area in Fraubrunnen as the main GMP clean room area and be sufficient for both locations.
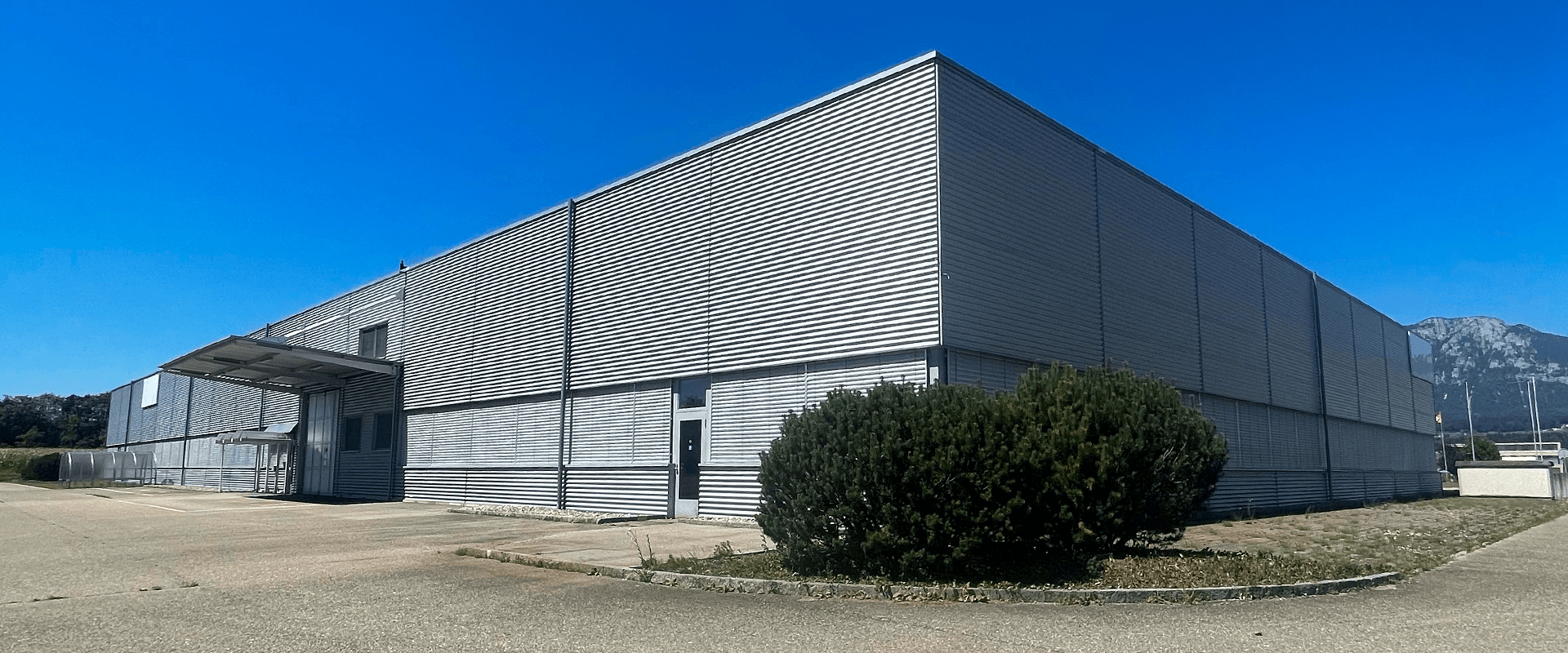
🌱 3) GMP location
Details of the new location (Solothurn, Luterbach):
In the course of 2022, we bought a plot of land with almost 10,000m² including a hall through our group structure.
You can see a picture of the new location below.
The hall is not intended for the sale of more plants, but only for the final GMP clean room area, as well as GMP further processing of medical cannabis products, and for another research center.
The planning for this is already in full swing and we expect to submit for the building permit in December 2022.
Construction should start after the building permit has been approved in early 2023 (Q2 2023), since building permits in the canton of Solothurn usually take about 3 months and we don’t have to install as much technology as at our two production sites.
The completion of the construction and the audit for the GMP certification should take place at the end of Q1 2024.
So we have 3 locations:
1. Berne, Fraubrunnen
2. Solothurn, Zuchwil
3. Solothurn, Luterbach

🌱 4) Sale of the last plants
By moving the GMP location from Solothurn to our third location, 5000 plants became available at the Solothurn location, which is why we changed the display in the Cannergrow backoffice from percentage to the amount of plants so that the number of sellable plants is transparent for everyone .
As previously communicated, we will stop selling plants after the grow room Zeus is sold out.
🌱 5) Laboratory area
Our laboratory area with our biology and biochemistry laboratory is currently being built at the Fraubrunnen site.
In addition to the GMP clean room area, the laboratory area will also receive GMP certification.
With our two laboratories, we carry out research projects and studies on the medical use of THC cannabis together with doctors and university hospitals.
Furthermore, our laboratory area is used to approve the internal batch release.
Approval means: Checking each harvest to ensure quality based on the EU-GMP regulation.
The laboratory area will be completed by the end of November. The laboratory devices are currently being installed and then validated and approved by the respective manufacturers.
We hope we were able to enlighten you with this extensive blog article about the current status of GMP certification and how close we are to our goal together.
#WeGrowForYou
Your Cannerald & CannerGrow Team